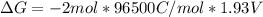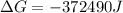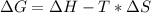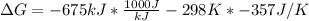Chemistry, 29.11.2019 06:31 Supermate11
Free-energy change, δg∘, is related to cell potential, e∘, by the equationδg∘=−nfe∘where n is the number of moles of electrons transferred and f=96,500c/(mol e−) is the faraday constant. when e∘ is measured in volts, δg∘ must be in joules since 1 j=1 c⋅v.1. calculate the standard free-energy change at 25 ∘c for the following reaction: mg(s)+fe2+(aq)→mg2+(aq)+fe(s)expres s your answer to three significant figures and include the appropriate units.2. calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘ = -675 kj and δs∘ = -357 j/k .express your answer to three significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal.b. he is determining chemical properties that are sufficient to identify the metal.c. he is determining physical properties that are insufficient to identify the metal.d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 23.06.2019 01:00
Which polymers are most closely related? a. protein and nucleic acids b. cellulose and starch c. nucleic acids and starch d. nucleic acids and cellulose
Answers: 2

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1
You know the right answer?
Free-energy change, δg∘, is related to cell potential, e∘, by the equationδg∘=−nfe∘where n is the nu...
Questions



Mathematics, 23.04.2020 06:17



Social Studies, 23.04.2020 06:18



Advanced Placement (AP), 23.04.2020 06:18


History, 23.04.2020 06:18

Mathematics, 23.04.2020 06:18


Biology, 23.04.2020 06:18


Mathematics, 23.04.2020 06:18

Social Studies, 23.04.2020 06:18

Mathematics, 23.04.2020 06:18


History, 23.04.2020 06:18

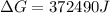
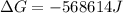
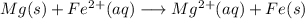
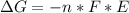
![E=E_{red}-E_{ox]](/tpl/images/0395/8514/34231.png)