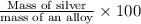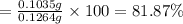Chemistry, 29.11.2019 05:31 SophieCasey
Asilver-copper alloy had a mass of 0.1264g. when the alloy was dissolved in nitric acid and the silver precipitated as silver chloride, the precipitate had a mass of 0.1375g. calculate the percent of silver in the alloy. for full credit, make sure you show your calculations.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
Will give brainliest it is a lab from k12 here is the linkfor each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. type your answer here. (score for question 3: of 5 points) were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. type your answer here. (score for question 4: of 5 points) make a general statement about the reactivity of the metals in this experiment. type your answer here.
Answers: 2

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3
You know the right answer?
Asilver-copper alloy had a mass of 0.1264g. when the alloy was dissolved in nitric acid and the silv...
Questions

History, 11.12.2019 19:31








English, 11.12.2019 19:31

Mathematics, 11.12.2019 19:31

Mathematics, 11.12.2019 19:31

Biology, 11.12.2019 19:31



History, 11.12.2019 19:31


Mathematics, 11.12.2019 19:31



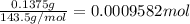
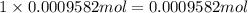 of silver
of silver