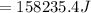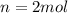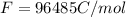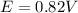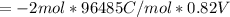Chemistry, 29.11.2019 04:31 thelukp7fihp
Calculate the standard free energy change as a pair of electrons is transferred from succinate to molecular oxygen in the mitochondrial respiratory chain. oxidant reductant n e∘′(v) fumarate 2h 2e− ⇌ succinate 2 0.03 12o₂ 2h 2e− ⇌ h2o 2 0.82 express your answer to two significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 16:20
When water dissolves sugar, which process is not involved? o dissociation o hydration o surface area of the solute increases sa
Answers: 1

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1
You know the right answer?
Calculate the standard free energy change as a pair of electrons is transferred from succinate to mo...
Questions

Chemistry, 12.07.2019 03:00

Chemistry, 12.07.2019 03:00


Mathematics, 12.07.2019 03:00

History, 12.07.2019 03:00




Spanish, 12.07.2019 03:00

Health, 12.07.2019 03:00

Geography, 12.07.2019 03:00





History, 12.07.2019 03:00