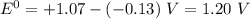Chemistry, 29.11.2019 00:31 savyblue1724707
Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25°c. (the equation is balanced.) pb(s) + br2(l) → pb2+(aq) + 2br(aq) pb2+(aq) + 2 e → pb(s) e° = -0.13 v br2(l) + 2 e → 2 br(aq) e° = +1.07 v

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which of the following would be an accurate picture of the earth during the summer time of the northern hemisphere?
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 10:00
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

You know the right answer?
Use the standard half-cell potentials listed below to calculate the standard cell potential for the...
Questions


Chemistry, 07.10.2019 03:00



Mathematics, 07.10.2019 03:00





Biology, 07.10.2019 03:00

English, 07.10.2019 03:00







Biology, 07.10.2019 03:00

Health, 07.10.2019 03:00

Biology, 07.10.2019 03:00

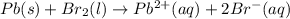
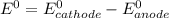
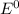 are standard reduction potentials.
are standard reduction potentials.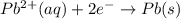
![E^0_{[Pb^{2+}/Pb]}= -0.13\ V](/tpl/images/0395/3322/82712.png)
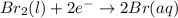
![E^0_{[Br_2/Br^{-}]}=+1.07\ V](/tpl/images/0395/3322/f8f7e.png)
![E^0=E^0_{[Br_2/Br^{-}]}- E^0_{[Pb^{2+}/Pb]}](/tpl/images/0395/3322/fba3a.png)