
Chemistry, 29.11.2019 00:31 CameronVand21
She dissolves a 10.0mg sample in enough water to make 30.0ml of solution. the osmotic pressure of the solution is 0.340torr at 25c. a). what is the molar mass of the gene fragment? b). if the solution density is 0.997g/ml, what is the freezing point for this aqueous solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium
Answers: 1

Chemistry, 23.06.2019 01:30
What is the importance of interlocking the fingers and rubbing while washing hands? the palms are the dirtiest parts of the hands. the spaces between the fingers get washed. the backs of the hands get washed. the fingernails are the dirtiest parts of the hands
Answers: 1

Chemistry, 23.06.2019 01:30
Witch two conditions can limit the usefulness of the kinetic molecular theory in describing gas behavior?
Answers: 2
You know the right answer?
She dissolves a 10.0mg sample in enough water to make 30.0ml of solution. the osmotic pressure of th...
Questions


Spanish, 18.05.2021 18:20


Mathematics, 18.05.2021 18:20

Mathematics, 18.05.2021 18:20

Mathematics, 18.05.2021 18:20

English, 18.05.2021 18:20



Mathematics, 18.05.2021 18:20

Mathematics, 18.05.2021 18:20



Mathematics, 18.05.2021 18:20

Mathematics, 18.05.2021 18:20




Mathematics, 18.05.2021 18:20

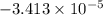 C
C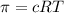
 = Concentration of solution
= Concentration of solution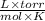
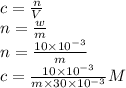
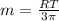
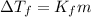
 mol/kg
mol/kg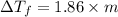
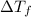 =
= 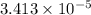 C
C

