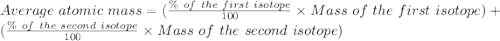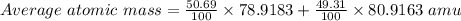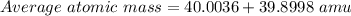Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2


Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
Bromine has two isotopes 79br and 81br, whose masses (78.9183 and 80.9163 amu) and abundances (50.69...
Questions




History, 29.09.2019 18:30


Social Studies, 29.09.2019 18:30

Biology, 29.09.2019 18:30

Social Studies, 29.09.2019 18:30




Health, 29.09.2019 18:30

Mathematics, 29.09.2019 18:30


English, 29.09.2019 18:30



Biology, 29.09.2019 18:30

English, 29.09.2019 18:30