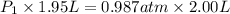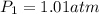Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:30
Calculate the concentration of all species in a 0.165 m solution of h2co3.
Answers: 1

Chemistry, 23.06.2019 00:00
Predict the relative bond lengths of the three carbon-oxygen bonds in the carbonate ion (co2−3). what would you expect the charge to be on each oxygen? match the words in the left column to the appropriate blanks in the sentences on the right. make certain each sentence is complete before submitting your answer.
Answers: 3

Chemistry, 23.06.2019 03:50
Show how to convert the temperature 84.7° c to kelvin. include all steps and label the final answer
Answers: 1

You know the right answer?
A2.00-l sample of was collected over water at a total pressure of 750. torr and 22 °c. when the was...
Questions




Mathematics, 26.12.2020 20:00

Mathematics, 26.12.2020 20:00


Mathematics, 26.12.2020 20:00

Mathematics, 26.12.2020 20:00










Physics, 26.12.2020 20:00


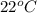 is 1.01 atm.
is 1.01 atm.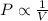
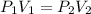
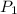 = initial pressure = ?
= initial pressure = ?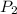 = final pressure = 750 torr = 0.987 atm (1 atm = 760 torr)
= final pressure = 750 torr = 0.987 atm (1 atm = 760 torr)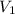 = initial volume = 1.95 L
= initial volume = 1.95 L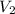 = final volume = 2.00 L
= final volume = 2.00 L