
Chemistry, 28.11.2019 22:31 leannadoughty06
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0-ml of 1 m h2so4. a 25.00 ml aliquot is analyzed by adding ki and titrating the i3– that forms with s2o32–. the end point was reached following the addition of 13.02 ml of 0.03428 m na2s2o3. calculate the weight percent of ce4 in the sample. when should the indicator be added to this titration? (at the beginning or just before the end point? )

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:00
Condensation happens when water vapor cools. true or false? ?
Answers: 2

Chemistry, 23.06.2019 06:30
When microscope slides are stained to show blood cells, the small red blood cells that appear on the slides are much numerous than the large white blood cells. this supports the concept that
Answers: 1

Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1

Chemistry, 23.06.2019 10:00
Which number should be placed before f2 on the reactants side equation to make equation balanced? xe + > xef4
Answers: 1
You know the right answer?
A3.452 g sample containing an unknown amount of a ce(iv) salt is dissolved in 250.0-ml of 1 m h2so4....
Questions

Chemistry, 21.03.2020 12:00

Mathematics, 21.03.2020 12:00



Health, 21.03.2020 12:01


History, 21.03.2020 12:01




Mathematics, 21.03.2020 12:01

Mathematics, 21.03.2020 12:01

English, 21.03.2020 12:01

Business, 21.03.2020 12:01


Mathematics, 21.03.2020 12:01


Social Studies, 21.03.2020 12:01


History, 21.03.2020 12:01

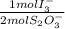 = 2,2315x10⁻⁴ moles of I₃⁻
= 2,2315x10⁻⁴ moles of I₃⁻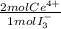 = 4,463x10⁻⁴ moles of Ce(IV). These moles are:
= 4,463x10⁻⁴ moles of Ce(IV). These moles are: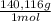 = 0,0625 g of Ce(IV)
= 0,0625 g of Ce(IV)

