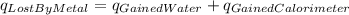In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used to determine the specific heat of a solid, or to measure the energy of a solution phase reaction. a student heats 61.68 grams of gold to 99.01 °c and then drops it into a cup containing 79.34 grams of water at 22.14 °c. she measures the final temperature to be 23.98 °c. the heat capacity of the calorimeter (sometimes referred to as the calorimeter constant) was determined in a separate experiment to be 1.80 j/°c. assuming that no heat is lost to the surroundings calculate the specific heat of gold.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

You know the right answer?
In the laboratory a "coffee cup" calorimeter, or constant pressure calorimeter, is frequently used t...
Questions

Biology, 22.06.2019 17:30






Mathematics, 22.06.2019 17:30


Mathematics, 22.06.2019 17:30





Mathematics, 22.06.2019 17:30

History, 22.06.2019 17:30

Computers and Technology, 22.06.2019 17:30



English, 22.06.2019 17:30