
Chemistry, 28.11.2019 19:31 bubster5820
Which substance is the limiting reactant when 4.0 g of sulfur reacts with 6.0 g of oxygen and 8.0 g of sodium hydroxide according to the following chemical
2 s(s) + 3 o2(g) + 4 naoh(aq) → 2 na2so4(aq) + 2 h2o(l)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 21:20
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2
You know the right answer?
Which substance is the limiting reactant when 4.0 g of sulfur reacts with 6.0 g of oxygen and 8.0 g...
Questions

History, 04.08.2019 21:00

History, 04.08.2019 21:00

Chemistry, 04.08.2019 21:00

Mathematics, 04.08.2019 21:00



Spanish, 04.08.2019 21:00

Biology, 04.08.2019 21:00

Computers and Technology, 04.08.2019 21:00


History, 04.08.2019 21:00



Biology, 04.08.2019 21:00

Biology, 04.08.2019 21:00

History, 04.08.2019 21:00

Biology, 04.08.2019 21:00

Chemistry, 04.08.2019 21:00

Mathematics, 04.08.2019 21:00

Biology, 04.08.2019 21:00

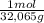 = 0,125 moles S
= 0,125 moles S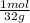 = 0,188 moles O₂
= 0,188 moles O₂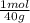 = 0,2 moles NaOH
= 0,2 moles NaOH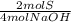 = 0,1 moles of Sulfur
= 0,1 moles of Sulfur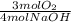 = 0,15 moles of Oxygen
= 0,15 moles of Oxygen

