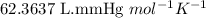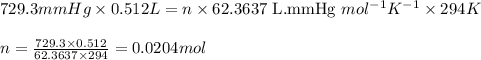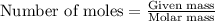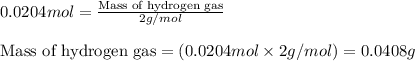Chemistry, 28.11.2019 19:31 snowprincess99447
Asample of hydrogen gas was collected over water at 21°c and at a pressure equal to the atmospheric pressure of 748 mm hg. the volume of the sample was 512 ml. how many grams of h2 are in the sample? the vapor pressure of water at 21°c is 18.7 mmhg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3

Chemistry, 22.06.2019 11:30
Which statement best describes the flow of energy in this scenario
Answers: 1

Chemistry, 22.06.2019 13:30
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
Asample of hydrogen gas was collected over water at 21°c and at a pressure equal to the atmospheric...
Questions

Mathematics, 15.04.2021 01:00


Chemistry, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00


History, 15.04.2021 01:00

History, 15.04.2021 01:00



Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00


Mathematics, 15.04.2021 01:00

Health, 15.04.2021 01:00

Mathematics, 15.04.2021 01:00

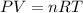
![21^oC=[21+273]K=294\ K](/tpl/images/0394/9433/09af2.png)