
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition of sodium azide, nan3. if an air bag has a volume of 43.8 l and is to be filled with nitrogen gas at a pressure of 1.13 atm at a temperature of 22.4˚c, how many moles of nan3 must decompose? you may assume the n2 behaves as an ideal gas. if carmen adds zeros behind the decimal place in your answer, do not worry. it will be graded correctly.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 21:00
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
The air bags in automobiles were once inflated by nitrogen gas generated by the rapid decomposition...
Questions

Computers and Technology, 17.07.2019 11:40


Chemistry, 17.07.2019 11:40

History, 17.07.2019 11:40

Biology, 17.07.2019 11:40

History, 17.07.2019 11:40

Biology, 17.07.2019 11:40

Mathematics, 17.07.2019 11:40

Mathematics, 17.07.2019 11:40

English, 17.07.2019 11:40

Computers and Technology, 17.07.2019 11:40


History, 17.07.2019 11:40


Social Studies, 17.07.2019 11:40





 = (22.4 + 273) K = 295.4 K
= (22.4 + 273) K = 295.4 K

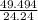
 must decompose.
must decompose.

