
Chemistry, 28.11.2019 06:31 mxltie1651
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide gas. you can assume both gases behave as ideal gases under these conditions. calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. round each of your answers to 3 significant digits. sulfur tetraflouride: mole fraction? partial pressure? carbon dioxide: mole fraction? partial pressure? total pressure in tank?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:30
Two metal blocks that have slightly different temperatures are placed next to one another. after five minutes, they both have lower but equal temperatures. according to the law of conservation of energy, what most likelyhappened? energy was created inside the blocks.energy was destroyed inside the blocks.energy was absorbed into the blocks from outside the system.energy was transferred from the warmer block to the cooler block.
Answers: 2

Chemistry, 23.06.2019 00:50
The chemical formula for emerald is be3al2(sio3)6.an emerald can be decided as
Answers: 3

Chemistry, 23.06.2019 02:30
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 03:00
Abaker touches a pie right after taking it out of the oven. which statement best explains why the pie feels hot?
Answers: 1
You know the right answer?
A9.000l tank at 27.0°c is filled with 5.29g of sulfur tetrafluoride gas and 15.6g of carbon dioxide...
Questions


History, 28.08.2020 20:01

Mathematics, 28.08.2020 20:01






Mathematics, 28.08.2020 20:01


English, 28.08.2020 20:01

Mathematics, 28.08.2020 20:01


Chemistry, 28.08.2020 20:01






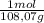 = 0,0489 moles SF₄
= 0,0489 moles SF₄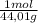 = 0,354 moles CO₂
= 0,354 moles CO₂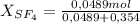 = 0,121
= 0,121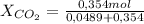 = 0,879
= 0,879


