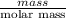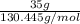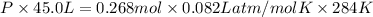Chemistry, 28.11.2019 05:31 cookies1164
Areaction between liquid reactants takes place at - 11.0 degree c in a sealed, evacuated vessel with a measured volume of 45.0 l. measurements show that the reaction produced 35. g of chlorine pentafluoride gas. calculate the pressure of chlorine pentafluoride gas in the reaction vessel after the reaction. you may ignore the volume of the liquid of reactants. be sure your answer has the correct number of significant digits.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 08:30
Draw the skeletal structures of two different molecules that are each made of 5 carbon atoms and 12 hydrogen atoms.
Answers: 1

Chemistry, 22.06.2019 17:10
Increasing the substrate concentration in an enzymatic reaction could overcome which of the following? a) the need for a coenzymeb) allosteric inhibitionc) competitive inhibitiond) insufficient cofactors
Answers: 1
You know the right answer?
Areaction between liquid reactants takes place at - 11.0 degree c in a sealed, evacuated vessel with...
Questions






Social Studies, 28.09.2020 04:01


Social Studies, 28.09.2020 04:01

Biology, 28.09.2020 04:01

Mathematics, 28.09.2020 04:01


Mathematics, 28.09.2020 04:01

Biology, 28.09.2020 04:01

Mathematics, 28.09.2020 04:01

English, 28.09.2020 04:01


Mathematics, 28.09.2020 04:01

Spanish, 28.09.2020 04:01

Mathematics, 28.09.2020 04:01

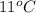 = (11 + 273) K = 284 K, V = 45.0 L
= (11 + 273) K = 284 K, V = 45.0 L