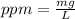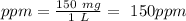Chemistry, 28.11.2019 05:31 jellyangie1
Astock solution contains a mixture of ~100 ppm chloride, fluoride, nitrite, bromide, nitrate and phosphate anions. in order to prepare 1 l of 100 ppm nitrite stock solution, you weigh out 150.0 mg of nano2. the actual concentration of nitrite would be:

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 12:30
The missing component to the table to the right or indicated with orange letters complete the table by filling in the corresponding numbers or symbols
Answers: 3

Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1
You know the right answer?
Astock solution contains a mixture of ~100 ppm chloride, fluoride, nitrite, bromide, nitrate and pho...
Questions

Mathematics, 16.04.2021 14:00




Mathematics, 16.04.2021 14:00




Mathematics, 16.04.2021 14:00

English, 16.04.2021 14:00

Mathematics, 16.04.2021 14:00

Physics, 16.04.2021 14:00

Business, 16.04.2021 14:00

History, 16.04.2021 14:00


English, 16.04.2021 14:00

Mathematics, 16.04.2021 14:00



History, 16.04.2021 14:00

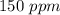
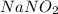 , so:
, so: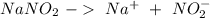
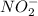 is 1:1
is 1:1