Chemistry, 28.11.2019 03:31 jaylabeatty44
Use the standard half-cell potentials listed below to calculate the standard cell potential for the following reaction occurring in an electrochemical cell at 25�c. (the equation is balanced.)
3 cl2(g) + 2 fe(s) --> 6 cl-(aq) + 2 fe3+(aq)
cl2(g) + 2 e- --> 2 cl-(aq); e� = +1.36 v
fe3+(aq) + 3 e- --> fe(s); e� = -0.04 v
+1.32 v
-1.32 v
-1.40 v
+1.40 v
+4.16 v

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Which of the following can be used to measure electricity
Answers: 1

Chemistry, 22.06.2019 04:00
14. many depressants reduce small muscle control, making it harder for a. you to steer b. your mind to consider complex problems c. the eye to scan, focus, or stay still d. the kidneys to filter alcohol out of the bloodstream
Answers: 3

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 14:00
Will mark brainliest how many electrons can be held in the energy level n = 4?
Answers: 1
You know the right answer?
Use the standard half-cell potentials listed below to calculate the standard cell potential for the...
Questions



Mathematics, 03.02.2021 16:50


Physics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50



English, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50

Biology, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50





Mathematics, 03.02.2021 16:50


Mathematics, 03.02.2021 16:50

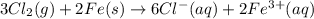
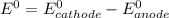
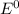 are standard reduction potentials.
are standard reduction potentials.
![E^0_{[Fe^{3+}/Fe]}=-0.04V](/tpl/images/0394/1963/07a45.png)
![E^0_{[Cl_2/Cl^-]}=+1.36V](/tpl/images/0394/1963/05c05.png)
![E^0=E^0_{[Cl_2/Cl^-]}- E^0_{[Fe^{3+}/Fe]}](/tpl/images/0394/1963/d357e.png)