
Chemistry, 28.11.2019 02:31 amanquen35
Find the equilibrium partial pressures of a and b for each of the following different values of kp.? consider the following reaction: a(g) = 2b(g)find the equilibrium partial pressures of a and b for each of the following different values of kp. assume that the initial partial pressure of b in each case is 1.0 atm and that the initial partial pressure of a is 0.0 atm. make any appropriate simplifying assumptions. kp = 1.4? kp = 2.0 * 10^-4? kp = 2.0 * 10^5?

Answers: 2


Another question on Chemistry


Chemistry, 23.06.2019 01:00
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2

Chemistry, 23.06.2019 05:10
Will mark as brainliest ! how many grams of iron metal do you expect to be produced when 245 grams of an 80.5 percent by mass iron (ii) nitrate solution react with excess aluminum metal? show all work needed to solve this problem!
Answers: 1

Chemistry, 23.06.2019 06:30
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1
You know the right answer?
Find the equilibrium partial pressures of a and b for each of the following different values of kp.?...
Questions





Social Studies, 18.03.2021 01:10


History, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Business, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


Mathematics, 18.03.2021 01:10


English, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10


English, 18.03.2021 01:10

Mathematics, 18.03.2021 01:10

![P_{[A] = 0,22](/tpl/images/0394/1164/9afd7.png) ,
, ![P_{[B] = 0,56atm](/tpl/images/0394/1164/02e2b.png)
![P_{[A] = 0,495](/tpl/images/0394/1164/54750.png) ,
, ![P_{[B] = 0,01atm](/tpl/images/0394/1164/da9a1.png)
![P_{[A] = 5x10^{-6}](/tpl/images/0394/1164/9be03.png) ,
, ![P_{[B] = 0,99999atm](/tpl/images/0394/1164/07800.png)
![kp = \frac{P_{[B]}^2}{P_{[A]}}](/tpl/images/0394/1164/0f2b8.png)
![P_{[A] = 0,0atm + X](/tpl/images/0394/1164/62221.png)
![P_{[B] = 1,0atm - 2X](/tpl/images/0394/1164/dd6ae.png)
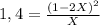
![P_{[B] = 1,0atm - 0,44atm = 0,56atm](/tpl/images/0394/1164/4f71b.png)
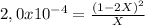
![P_{[A] = 0,495atm](/tpl/images/0394/1164/e1edd.png)
![P_{[B] = 1,0atm - 0,99atm = 0,01atm](/tpl/images/0394/1164/aeba4.png)
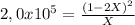
![P_{[B] = 1,0atm - 0,00001atm = 0,99999atm](/tpl/images/0394/1164/ca009.png)


