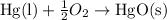Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 18:30
When the chemicals iron sulfide (fes) and hydrochloric acid (hcl) are combined, bubbles appear from the mixture. 1. does the appearance of bubbles indicate a physical or chemical change? 2. why do the bubbles indicate this change? 3. what property is this?
Answers: 1
You know the right answer?
When 18.5 g of hgo(s) is decomposed to form hg(l) and o2(g), 7.75 kj of heat is absorbed at standard...
Questions


Mathematics, 26.02.2021 22:50


Mathematics, 26.02.2021 22:50

Spanish, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50


Mathematics, 26.02.2021 22:50

Biology, 26.02.2021 22:50


History, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50


English, 26.02.2021 22:50

Mathematics, 26.02.2021 22:50


Biology, 26.02.2021 22:50