Chemistry, 27.11.2019 22:31 wardlawshaliyah
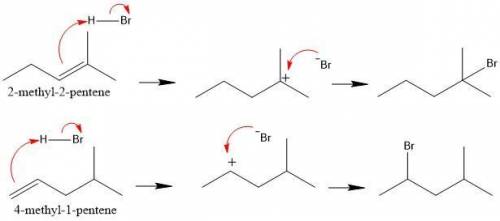
Identify which of the following two reactions you would expect to occur more rapidly: (1) addition of hbr to 2-methyl-2-pentene or (2) addition of hbr to 4-methyl-1-pentene. explain your choice. addition of hbr to should be more rapid because the reaction can proceed via a carbocation. in contrast, addition of hbr to proceeds via a less stable, carbocation.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:10
Precision can be defined as the o exact center of a data set. o reproducibility of a measured value. o correlation between two variables that are measured in a data set agreement between a measured value and an accepted value.
Answers: 2

Chemistry, 22.06.2019 15:00
Which of the following is the correct formula for copper (i) sulfate trihydrate? cuso4 · 3h2o cuso4(h2o)3 cu2so4(h2o)3 cu2so4 · 3h2o
Answers: 1


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Identify which of the following two reactions you would expect to occur more rapidly: (1) addition...
Questions


Mathematics, 13.04.2021 19:20

World Languages, 13.04.2021 19:20

Mathematics, 13.04.2021 19:20

Mathematics, 13.04.2021 19:20

Mathematics, 13.04.2021 19:20

English, 13.04.2021 19:20

History, 13.04.2021 19:20


Mathematics, 13.04.2021 19:20

Biology, 13.04.2021 19:20




Mathematics, 13.04.2021 19:20

Social Studies, 13.04.2021 19:20

History, 13.04.2021 19:20

Mathematics, 13.04.2021 19:20


Mathematics, 13.04.2021 19:20