
Chemistry, 27.11.2019 22:31 keishadawson
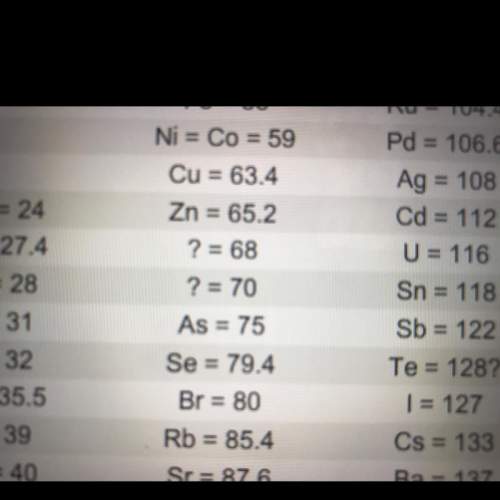
look at the two question marks between zinc (zn) and arsenic (as). at the time, no elements were known with atomic weights between 65.2 and 75. but mendeleev predicted that two elements must exist with atomic weights in this range.
what led mendeleev to predict that two undiscovered elements existed in that range?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1


You know the right answer?
look at the two question marks between zinc (zn) and arsenic (as). at the time, no elements were kno...
Questions

Mathematics, 26.09.2019 05:30

English, 26.09.2019 05:30



Business, 26.09.2019 05:30

English, 26.09.2019 05:30




History, 26.09.2019 05:30




Chemistry, 26.09.2019 05:30




English, 26.09.2019 05:30





