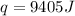Chemistry, 27.11.2019 20:31 destinycolley728
How much heat do you need to raise the temperature of 150 g of ice from −30 °c to −15 °c ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 22.06.2019 08:00
Will give ! what are the advantages and disadvantages of nuclear power? check all that apply. one advantage of nuclear energy is that it does not produce carbon dioxide emissions. storage of nuclear waste is a short-term problem associated with nuclear energy. the problem with uranium mining is that a large quantity of uranium must be extracted to meet energy needs because the energy release from uranium fission is so low. safe operation of a nuclear power plant can be jeopardized by a human mistake.
Answers: 1
You know the right answer?
How much heat do you need to raise the temperature of 150 g of ice from −30 °c to −15 °c ?...
Questions

Mathematics, 17.12.2020 06:40


Health, 17.12.2020 06:40


World Languages, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40





Mathematics, 17.12.2020 06:40

Arts, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40


Mathematics, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40

Mathematics, 17.12.2020 06:40

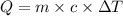
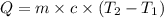
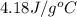
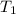 = initial temperature =
= initial temperature = 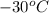
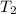 = final temperature =
= final temperature = 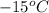
![q=150g\times 4.18J/g^oC\times [(-15)-(-30)]^oC](/tpl/images/0393/5324/8f79f.png)