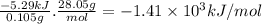Chemistry, 27.11.2019 19:31 billyeyelash
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was burned in this calorimeter, the temperature increased by 2.14 k. calculate the energy of combustion for one mole of ethylene. a. –1.41 × 103 kj/mol b. –660 kj/mol c. –5.29 kj/mol d. –0.259 kj/mol e. –50.3 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:00
Which of the following is a physical property flammability heat of combustion solubility and toxicity
Answers: 1

Chemistry, 22.06.2019 22:00
The diagrams to the right show the distribution and arrangement of gas particles in two different containers. according to kinetic-molecular theory, which of the following statements is true? check all that apply. if the temperatures of both containers are equal, container a has greater pressure than container b. if the volume of container a decreased, its pressure would decrease. if the pressure in both containers is equal, container a has a lower temperature than container b.
Answers: 2

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 10:30
How much mass would a mole of hydrogen molecules contain? recall that hydrogen is diatomic. g/mol
Answers: 3
You know the right answer?
Abomb calorimeter has a heat capacity of 2.47 kj/k. when a 0.105-g sample of ethylene (c2h4) was bur...
Questions

Mathematics, 18.10.2020 04:01


Mathematics, 18.10.2020 04:01

History, 18.10.2020 04:01


English, 18.10.2020 04:01

Mathematics, 18.10.2020 04:01








Mathematics, 18.10.2020 04:01


Mathematics, 18.10.2020 04:01

English, 18.10.2020 04:01

Mathematics, 18.10.2020 04:01