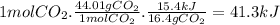Chemistry, 27.11.2019 19:31 eheheh80ii
The reaction of carbon dioxide(g) with hydrogen(g) to form carbon monoxide(g) and water(g) proceeds as follows: co2(g) + h2(g) > co(g) + h2o(g)when 16.4 grams of co2(g) react with sufficient h2(g) , 15.4 kj of energy areabsorbed .what is the value of > h for the chemical equation given? δhrxn = kj

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 09:50
What are four significant sources of ghgs that come from wostem washington?
Answers: 2


Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
The reaction of carbon dioxide(g) with hydrogen(g) to form carbon monoxide(g) and water(g) proceeds...
Questions

Mathematics, 06.05.2020 08:10


Chemistry, 06.05.2020 08:10


Business, 06.05.2020 08:10

Geography, 06.05.2020 08:10

English, 06.05.2020 08:10


English, 06.05.2020 08:10

Mathematics, 06.05.2020 08:10


Social Studies, 06.05.2020 08:10


English, 06.05.2020 08:10



Mathematics, 06.05.2020 08:10


Mathematics, 06.05.2020 08:10

Social Studies, 06.05.2020 08:10