
Chemistry, 27.11.2019 06:31 mdaniella522
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standard free energy change(deltago’) for this reaction is +1.7 kj/mol. if the cellular concentrations are 2pg = 0.5 mm and pep = 0.1 mm, what is the free energy change at 37 oc for the reaction 2pg ↔ pep? (a) 5.8 kj/mol(b) -5.8 kj/mol(c) +2.4 kj/mol(d) -2.4 kj/mol(e) -4146.4 kj/mol(f) +4146.4 kj/mol

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2


Chemistry, 22.06.2019 17:00
The biosphere of the earth is made up of what compound? organic or inorganic?
Answers: 3
You know the right answer?
2-phosphoglycerate(2pg) is converted to phosphoenolpyruvate (pep) by the enzyme enolase. the standar...
Questions





Mathematics, 20.02.2021 07:10

Mathematics, 20.02.2021 07:10

Chemistry, 20.02.2021 07:10

Mathematics, 20.02.2021 07:10



Chemistry, 20.02.2021 07:20

Mathematics, 20.02.2021 07:20

Physics, 20.02.2021 07:20


World Languages, 20.02.2021 07:20

World Languages, 20.02.2021 07:20




Mathematics, 20.02.2021 07:20

![Q_{r} =\frac{\left [ PEP \right ]}{\left [ 2PG \right ]} = \frac{0.1 mM}{0.5 mM} = 0.2](/tpl/images/0392/9257/2f0c6.png)
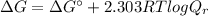
![\Delta G = 1.7 kJ/mol + [2.303 \times (8.314 \times 10^{-3} kJ/(K.mol))\times (310.15 K)] log (0.2)](/tpl/images/0392/9257/2a926.png)
![\Delta G = 1.7 + [5.938] \times (-0.699) = 1.7 - 4.15 = (-2.45 kJ/mol)](/tpl/images/0392/9257/65b39.png)


