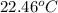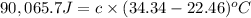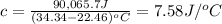Chemistry, 27.11.2019 06:31 lineaeriksen
A2.15g sample of benzene (c_6h_6) is burned in a bomb calorimeter, and the temperature rises from 22.46 degree c to 34.34 degree c. calculate the heat capacity of the bomb calorimeter. note the following thermochemical equation: c_6h_6(i) + 15/2 o_2 (g) rightarrow 6co_2 (g) + 3h_2o (g) delta h degree = -3267.5 kj

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Based on the equation and the information in the table, what is the enthalpy of the reaction?
Answers: 2

Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 09:10
When a nucleus absorbs a neutron and then breaks apart, there are many products of the reaction. what is not a product of a nuclear fission reaction
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
A2.15g sample of benzene (c_6h_6) is burned in a bomb calorimeter, and the temperature rises from 22...
Questions



Mathematics, 16.02.2021 18:50


Mathematics, 16.02.2021 18:50


Mathematics, 16.02.2021 18:50


Mathematics, 16.02.2021 18:50

Computers and Technology, 16.02.2021 18:50

Mathematics, 16.02.2021 18:50

Mathematics, 16.02.2021 18:50

Biology, 16.02.2021 18:50


Mathematics, 16.02.2021 18:50

Chemistry, 16.02.2021 18:50

Biology, 16.02.2021 18:50



History, 16.02.2021 18:50

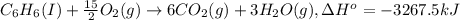
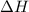 = enthalpy change = -3267.5 kJ/mol
= enthalpy change = -3267.5 kJ/mol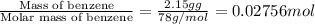
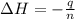
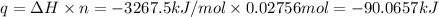
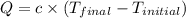
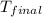 = final temperature =
= final temperature = 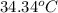
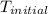 = initial temperature =
= initial temperature =