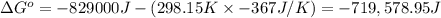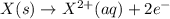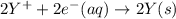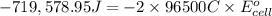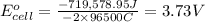Chemistry, 27.11.2019 04:31 rostecorralmart
Calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘ = -829 kj and δs∘ = -367 j/k

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:30
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3


Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 1
You know the right answer?
Calculate the standard cell potential at 25 ∘c for the reactionx(s)+2y+(aq)→x2+(aq)+2y(s)w here δh∘...
Questions





History, 18.04.2020 01:23


Chemistry, 18.04.2020 01:23


English, 18.04.2020 01:23



Mathematics, 18.04.2020 01:24

Mathematics, 18.04.2020 01:24




Computers and Technology, 18.04.2020 01:24


Social Studies, 18.04.2020 01:24

Mathematics, 18.04.2020 01:24


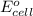 = standard electrode potential of the cell
= standard electrode potential of the cell