
Chemistry, 27.11.2019 04:31 johnny2585
What is the theoretical yield of vanadium, in moles, that can be produced by the reaction of 1.0 mole of v2o5 with 4.0 moles of calcium based on the following chemical equation? v2o5(s) + 5ca(l) → 2v(l) + 5cao(s)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
98 ! and brainliest plz ! the below reaction can be categorized as more than one type of reaction. which reactions are these, and what are the types of reactions?
Answers: 1

Chemistry, 23.06.2019 01:50
Ablock of aluminum is dropped into a graduated cylinder with an initial volume of water at 75ml and the volumes rises to 90ml. if the block has a mass of 40.5 g what is its density ?
Answers: 1

Chemistry, 23.06.2019 15:00
20 look at the clock and the data table below. based on the data and on your knowledge of potential and kinetic energy, what is the best conclusion you can make about potential and kinetic energy? the total amount of energy stays the same. the clock has the most potential energy at point b since it is moving the fastest. there is always more potential energy than kinetic energy. potential energy can never be 0, but you can have 0 kinetic energy.
Answers: 1

You know the right answer?
What is the theoretical yield of vanadium, in moles, that can be produced by the reaction of 1.0 mol...
Questions

Mathematics, 25.05.2021 23:20

History, 25.05.2021 23:20


Mathematics, 25.05.2021 23:20





Mathematics, 25.05.2021 23:20

Mathematics, 25.05.2021 23:20

Health, 25.05.2021 23:20


Social Studies, 25.05.2021 23:20

Mathematics, 25.05.2021 23:20



Chemistry, 25.05.2021 23:20

History, 25.05.2021 23:20

Biology, 25.05.2021 23:20

Social Studies, 25.05.2021 23:20

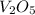 = 1.0 moles
= 1.0 moles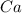 = 4.0 moles
= 4.0 moles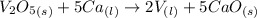
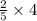 mole of V
mole of V

