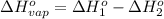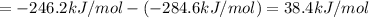Chemistry, 27.11.2019 03:31 KariSupreme
At a given set of conditions, 246.2 kj is given off when 1 mol of h2o(g) forms from its elements. under the same conditions, 284.6 kj is given off when 1 mol of h2o(l) forms from its elements. find δh for the vaporization of water at these conditions.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 23.06.2019 01:30
How does the attraction between particles affect the ability of a solvent to dissolve in a substance
Answers: 1

Chemistry, 23.06.2019 04:20
Malia was able to make a paper clip float on the surface of water what will most likely happen to the paper clip if a drop of dishwashing detergent is added near it
Answers: 1

You know the right answer?
At a given set of conditions, 246.2 kj is given off when 1 mol of h2o(g) forms from its elements. un...
Questions

Mathematics, 05.12.2020 03:10


Biology, 05.12.2020 03:10

Biology, 05.12.2020 03:10

Biology, 05.12.2020 03:10



Mathematics, 05.12.2020 03:10


English, 05.12.2020 03:10

Mathematics, 05.12.2020 03:10

Mathematics, 05.12.2020 03:10




History, 05.12.2020 03:10

Chemistry, 05.12.2020 03:10


Mathematics, 05.12.2020 03:10

Mathematics, 05.12.2020 03:10

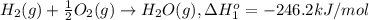 ..[1]
..[1]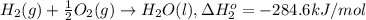 ..[2]
..[2]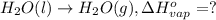 ...[3]
...[3]