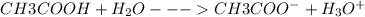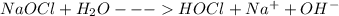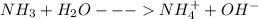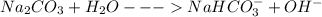Chemistry, 27.11.2019 03:31 xMABRYx1991
Write net brønsted equations that show the acidic or basic nature of the following solutions in water. for polyprotic species (vitamin c, lemon juice, and washing soda), show only one proton transfer. remember that spectator ions are not included. (use the lowest possible coefficients. omit states-of-matter in your answer.)
(a) vinegar (acetic acid, hc2h3o2)
(b) bleach (sodium hypochlorite, naocl)
(c) ammonia (nh3)
(d) vitamin c (ascorbic acid, h2c6h6o6)
(e) lemon juice (citric acid, h3c6h5o7)
(f) washing soda (sodium carbonate, na2co3)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 09:30
Based on its chemical properties, identify the position of each chemical family on the periodic table.
Answers: 3

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Write net brønsted equations that show the acidic or basic nature of the following solutions in wate...
Questions



Business, 26.09.2019 21:30





English, 26.09.2019 21:30



Physics, 26.09.2019 21:30

History, 26.09.2019 21:30



Mathematics, 26.09.2019 21:30


History, 26.09.2019 21:30

Mathematics, 26.09.2019 21:30


Biology, 26.09.2019 21:30