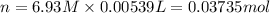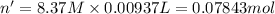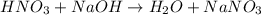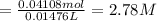Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is added to 9.37ml of 8.37m naoh. note the final volume is the sum of two added volumes. which of the following statement is true for the solution after mixing? a) naoh is in excess overhno3b)hno3 is in excess over naohc)hno3 and naoh are exactly balanced. what is the concentration of the excess naoh (or hno3) you indicated above?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 06:30
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 10:30
Consider the following reactions. (note: (s) = solid, (l) = liquid, and (g) = gas.) mg(s) + ½o2(g) → mgo(s) + 146 kcal/mole h2(g) + ½o2(g) → h2o(g), δh = -57.82 kcal/mole what type of reaction is represented by the previous two examples?
Answers: 3

Chemistry, 22.06.2019 13:30
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Calculate the concentration of acid(or base) remaining in solution when 5.39ml of 6.93m hno3 is adde...
Questions




Chemistry, 09.07.2019 02:00

English, 09.07.2019 02:00

History, 09.07.2019 02:00



History, 09.07.2019 02:00


Biology, 09.07.2019 02:00


Health, 09.07.2019 02:00

Biology, 09.07.2019 02:00

Mathematics, 09.07.2019 02:00


Spanish, 09.07.2019 02:00

English, 09.07.2019 02:00


Mathematics, 09.07.2019 02:00

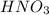 .
.