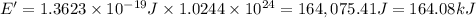Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
The reaction q+r2=r2q is found to be first order in r2 and
Answers: 1


Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Determine the energy of 2.00 mol of photons for each of the following kinds of light. (assume three...
Questions

Social Studies, 03.06.2021 15:10

Mathematics, 03.06.2021 15:20


Mathematics, 03.06.2021 15:20

Mathematics, 03.06.2021 15:20





Mathematics, 03.06.2021 15:20


Biology, 03.06.2021 15:20

Biology, 03.06.2021 15:20

SAT, 03.06.2021 15:20


Mathematics, 03.06.2021 15:20

Mathematics, 03.06.2021 15:20

Business, 03.06.2021 15:20

Computers and Technology, 03.06.2021 15:20

Computers and Technology, 03.06.2021 15:20

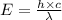
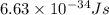
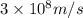
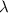 = wavelength =
= wavelength = 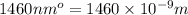
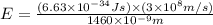
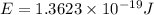
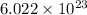 particles
particles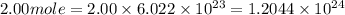 photons
photons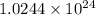 photons = E'
photons = E'