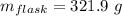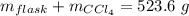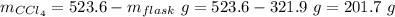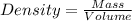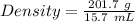Chemistry, 27.11.2019 02:31 Jakyramason
Aflask with a mass of 321.9 g is filled with 15.7 ml of carbon tetrachloride. the mass of the flask and carbon tetrachloride is found to be 523.6 g. from this information, calculate the density of carbon tetrachloride. according to this problem, the density of ccl4 is answer g/ml.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1

Chemistry, 22.06.2019 22:30
What is the value of the standard enthalpy of formation of an element in its most stable form?
Answers: 3

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1

Chemistry, 23.06.2019 05:00
Which characteristics affect ocean water’s temperature? check all that apply. depth location mass salinity waves
Answers: 1
You know the right answer?
Aflask with a mass of 321.9 g is filled with 15.7 ml of carbon tetrachloride. the mass of the flask...
Questions

Mathematics, 21.01.2021 14:10


History, 21.01.2021 14:20

Biology, 21.01.2021 14:20



Mathematics, 21.01.2021 14:20


Social Studies, 21.01.2021 14:20

Mathematics, 21.01.2021 14:20

Social Studies, 21.01.2021 14:20


Chemistry, 21.01.2021 14:20

Mathematics, 21.01.2021 14:20

Mathematics, 21.01.2021 14:20

Biology, 21.01.2021 14:20


Mathematics, 21.01.2021 14:20

Mathematics, 21.01.2021 14:20

Mathematics, 21.01.2021 14:20