
Chemistry, 27.11.2019 02:31 emilyy4757
Be sure to answer all parts. write a balanced equation and kb expression for the following brønsted-lowry base in water: benzoate ion, c6h5coo−. include the states of all reactants and products in your equation. you do not need to include states in the equilibrium expression. balanced equation: ⇌ kb expression:

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
Which of the following might a chemist choose to study? a. glacier movement in alaska b. better ways to recycle plastics c. the effects of hurricanes on turtle populations d. the vibrations in bridges caused by big trucks
Answers: 2

Chemistry, 22.06.2019 04:00
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1


Chemistry, 23.06.2019 06:40
The combustion of methane, ch4, releases 890.4kj/mol. that is, when one mole of methane is burned,890.4 kj are given off to the surroundings. this meansthat the products have 890.4 kj less than the reactants.thus, ah for the reaction = - 890.4 kj. a negative symbolforah indicates an exothermic reaction.ch (g) + 20 (g)> co2 (g) + 2 h0 (1); ah = - 890.4 kga) how much energy is given off when 2.00 mol of ch,are burned? b) how much energy is released when 22.4g of ch. areburned?
Answers: 1
You know the right answer?
Be sure to answer all parts. write a balanced equation and kb expression for the following brønsted-...
Questions

Mathematics, 11.03.2022 14:00

English, 11.03.2022 14:00

History, 11.03.2022 14:00






Mathematics, 11.03.2022 14:00

Mathematics, 11.03.2022 14:00

Mathematics, 11.03.2022 14:00



Mathematics, 11.03.2022 14:00

English, 11.03.2022 14:00

Mathematics, 11.03.2022 14:00

Mathematics, 11.03.2022 14:00

Mathematics, 11.03.2022 14:00


English, 11.03.2022 14:00

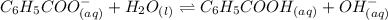
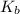 expression is:-
expression is:-![K_{b}=\frac {\left [ C_6H_5COOH \right ]\left [ {OH}^- \right ]}{[C_6H_5COO^-]}](/tpl/images/0392/5825/392ce.png)


