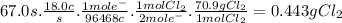Chemistry, 27.11.2019 01:31 ashhull2002
In the industrial "chlor-alkali" process, pure chlorine and sodium hydroxide are produced by electrolyzing brine, essentially an aqueous solution of sodium chloride.
suppose a current of 18.0 a is passed through an aqueous solution of nacl for 67.0 seconds.
calculate the mass of pure chlorine produced.
be sure your answer has a unit symbol and the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
When the water vapor cools it condenses select a number that represents his process on the
Answers: 3


Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 22:00
Plz ill give u brainliest which of the following steps is not likely to take place during cellular respiration? a.oxygen combines with carbon of simple sugar. b. energy molecule transfers energy to cells. d. energy is used up.
Answers: 3
You know the right answer?
In the industrial "chlor-alkali" process, pure chlorine and sodium hydroxide are produced by electro...
Questions

Mathematics, 01.09.2020 22:01

History, 01.09.2020 22:01

Mathematics, 01.09.2020 22:01



Social Studies, 01.09.2020 22:01


Mathematics, 01.09.2020 22:01

French, 01.09.2020 22:01

English, 01.09.2020 22:01

Mathematics, 01.09.2020 22:01




Mathematics, 01.09.2020 22:01

Biology, 01.09.2020 22:01

Mathematics, 01.09.2020 22:01



Mathematics, 01.09.2020 22:01