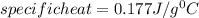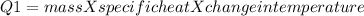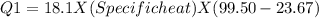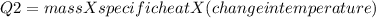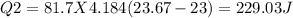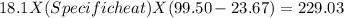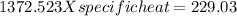Chemistry, 27.11.2019 01:31 sheram2010
In the laboratory, a student uses a coffee cup calorimeter to determine the specific heat of a metal. she heats 18.1 grams of lead to 99.50°c and then drops it into a cup containing 81.7 grams of water at 23.00°c. she measures the final temperature to be 23.67°c. assuming that all of the heat is transferred to the water, she calculates the specific heat of lead to be j/g°c.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
In the laboratory, a student uses a coffee cup calorimeter to determine the specific heat of a metal...
Questions

Mathematics, 17.12.2019 00:31



Social Studies, 17.12.2019 00:31


Physics, 17.12.2019 00:31






Geography, 17.12.2019 00:31








Social Studies, 17.12.2019 00:31