Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:10
Which statement is true about the part of the electromagnetic spectrum that human eyes can detect? it contains only the colors of the rainbow and television waves. o it is divided into seven ranges of wavelengths. it contains ultraviolet, visible, and infrared light. it is divided into nine ranges of wavelengths.
Answers: 2

Chemistry, 22.06.2019 08:30
Analyze how limestone is weathered and identify the features that are formed as a result of this dissolution
Answers: 1


You know the right answer?
Thermal decomposition of a rail car load of limestone to lime and carbon dioxide requires 2.97 x 106...
Questions





History, 25.06.2019 13:00

Health, 25.06.2019 13:00








Mathematics, 25.06.2019 13:00






English, 25.06.2019 13:00

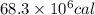
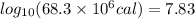
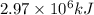
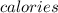 by using conversion factor:
by using conversion factor: