Chemistry, 26.11.2019 17:31 lanettejohnson355
Using the following data, determine the standard cell potential e^o cell for the electrochemical cell constructed using the following reaction, where zinc is the anode and lead is the cathode.
zn(s) + pb2+(aq) -> zn2+(aq) + pb(s)
half-reaction: standard reduction potential:
zn2+(aq) + 2e- -> zn(s)= -0.763
pb2+(aq) + 2e- -> pb(s)= -0.126
a. -0.889 v
b. +0.889 v
c. +0.637 v
d. +1.274 v
e. -0.637 v

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Balance and in which category does it fit in? single or double displacement or synthesis or decomposition? (a) k2 o → k + o2 (b) na + i2 → nai (c) cu(no3 )2 + naoh → cu(oh)2 + nano3 (d) kclo3 → kcl + o2 (e) ca(no3 )2 + hbr → cabr2 + hno3 (f) sn(oh)2 → sno + h2 o (g) p4 + n2 o → p4 o6 + n2 (h) fe + al2 (so4 )3 → feso4 + al (i) alcl3 + na2 co3 → al2 (co3 )3 + nacl (j) c3 h6 + o2 → co2 + h2 o
Answers: 1



Chemistry, 23.06.2019 03:50
Which best describes the activation energy of a chemical reaction? a. the combined energy of all the reactants b. the amount of energy required for a reaction to occur c. the difference in energy between products and reactants d. the potential energy stored in the bonds of reactants and products
Answers: 1
You know the right answer?
Using the following data, determine the standard cell potential e^o cell for the electrochemical cel...
Questions


Mathematics, 23.07.2020 23:01




Mathematics, 23.07.2020 23:01




Mathematics, 23.07.2020 23:01


Mathematics, 23.07.2020 23:01


Mathematics, 23.07.2020 23:01


Mathematics, 23.07.2020 23:01

Mathematics, 23.07.2020 23:01



Mathematics, 23.07.2020 23:01

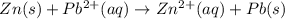
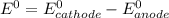
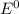 are standard reduction potentials.
are standard reduction potentials.
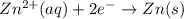 = -0.763
= -0.763
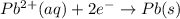 = -0.126
= -0.126![E^0_{[Zn^{2+}/Zn]}=-0.763V](/tpl/images/0391/6528/6b929.png)
![E^0_{[Pb^{2+}/Pb]}=-0.126V](/tpl/images/0391/6528/96123.png)
![E^0=E^0_{[Pb^{2+}/Pb]}- E^0_{[Zn^{2+}/Zn]}](/tpl/images/0391/6528/a01eb.png)