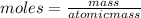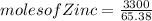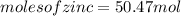Chemistry, 26.11.2019 07:31 loredohome
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant reaction is
zn2+(aq)+2e−→zn(s)
for a large batch of nails, a manufacturer needs to plate a total zinc mass of 3.30 kg on the surface to get adequate coverage.
part a
how many moles of zinc are in 3.30 kg of zinc?
express your answer to three significant figures and include the appropriate units.
50.5 mol
submithintsmy answersgive upreview part
correct
significant figures feedback: your answer 50.47mol was either rounded differently or used a different number of significant figures than required for this part.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Which is a character of nuclear fusion but not nuclear fission
Answers: 3

Chemistry, 21.06.2019 21:00
Write the chemical symbols for three different atoms or atomic cations with 27 electrons. asap!
Answers: 2

Chemistry, 21.06.2019 23:00
Matches the chemical name of each oxide of phosphorus to its chemical formula
Answers: 2

Chemistry, 23.06.2019 02:00
Pinene is an unsaturated hydrocarbon found in pine resin. if pinene has m+ = 136 and contains 1 double bond(s) and 2 ring(s); what is its molecular formula? enter the formula in the form ch first, then all other atoms in alphabetical order; do not use subscripts. the formula is case-sensitive
Answers: 3
You know the right answer?
Galvanized nails are iron nails that have been plated with zinc to prevent rusting. the relevant rea...
Questions

Mathematics, 06.02.2022 09:00

History, 06.02.2022 09:00

Mathematics, 06.02.2022 09:00

Biology, 06.02.2022 09:10


Chemistry, 06.02.2022 09:10


Mathematics, 06.02.2022 09:10


Mathematics, 06.02.2022 09:10

Business, 06.02.2022 09:10

Mathematics, 06.02.2022 09:10

Mathematics, 06.02.2022 09:10

Chemistry, 06.02.2022 09:10

Spanish, 06.02.2022 09:10


Biology, 06.02.2022 09:10


Mathematics, 06.02.2022 09:10

History, 06.02.2022 09:10