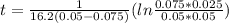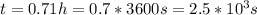a second- order reaction of the type a + b --> p was carried out in a solution that was initially 0.075 mol dm^-3 in a and 0.050 mol dm^-3 in b. after 1.0 h the concentration of a had fallen to 0.020 mol dm^-3. a) calculate the rate constant. b) solve for the half- life of each of the reactants.
hint: answers are a) 16.2 dm^3/mol*h
b) 5.1 × 10^3 s, 2.1 × 10^3 s

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 09:40
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. an industrial chemist studying this reaction fills a 25.0l tank with 4.5 mol of sulfur dioxide gas and 4.5 mol of oxygen gas at 30.°c. he then raises the temperature, and when the mixture has come to equilibrium measures the amount of sulfur trioxide gas to be 1.4 mol. calculate the concentration equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 3


Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
a second- order reaction of the type a + b --> p was carried out in a solution that was initially...
Questions

Mathematics, 24.03.2021 18:10

Computers and Technology, 24.03.2021 18:10


Mathematics, 24.03.2021 18:10


Spanish, 24.03.2021 18:10


English, 24.03.2021 18:10

English, 24.03.2021 18:10

Mathematics, 24.03.2021 18:10

English, 24.03.2021 18:10




Mathematics, 24.03.2021 18:10



Social Studies, 24.03.2021 18:10

Arts, 24.03.2021 18:10

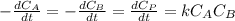
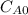 and
and 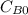 are the inital concentrations and x the concentration reacted at time t, so
are the inital concentrations and x the concentration reacted at time t, so 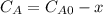 and
and 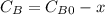 and the rate at time t is written as:
and the rate at time t is written as: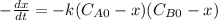
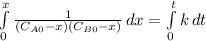
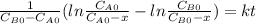
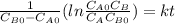
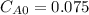 ,
, 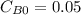 ,
,  , it implies that the quantity reacted, x, is 0.03 and
, it implies that the quantity reacted, x, is 0.03 and 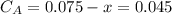 . Then, the value of k would be
. Then, the value of k would be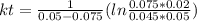
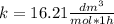
 so
so 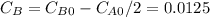 , k=16.2 and the same initial concentrations. Replacing in the equation
, k=16.2 and the same initial concentrations. Replacing in the equation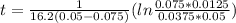
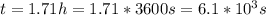
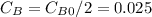 so
so 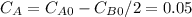 , k=16.2 and the same initial concentrations. Replacing in the equation
, k=16.2 and the same initial concentrations. Replacing in the equation