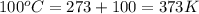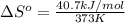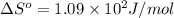Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
12. complete each of the following word equations for synthesis reactions. a. sodium + oxygen -> b. magnesium + fluorine -> 13. complete and balance the equations for the decomposition reactions. a. hgo -> [with the triangle heat symbol above the arrow] b. h2o(l) -> [with "electricity" written above the arrow]
Answers: 1

Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 08:00
Match the vocabulary terms to their definitions. 1 . a long, chain-like set of molecules made up of repeating units joined end to end polymer 2 . a hard, brittle, heat- and corrosion-resistant material made by subjecting a nonmetallic mineral mixture to intense heat ceramic 3 . a plastic with low elongations that cannot be recycled thermoset 4 . a carbon fiber embedded in a polymer resin matrix thermoplastic 5 . a plastic with high elongations that can be recycled crystal 6 . a solid form resulting from the arrangement of atoms, ions, or molecules in definite geometric patterns composite
Answers: 1

Chemistry, 23.06.2019 10:00
How to draw a diagram to represent a calcium metal lattice?
Answers: 3
You know the right answer?
For water ∆h°vap = 40.7 kj/mol at 100.°c, its boiling point. calculate ∆s° for the vaporization of 1...
Questions

Mathematics, 01.07.2019 16:00


Biology, 01.07.2019 16:00





Mathematics, 01.07.2019 16:00



Mathematics, 01.07.2019 16:00




Computers and Technology, 01.07.2019 16:00

Mathematics, 01.07.2019 16:00



Mathematics, 01.07.2019 16:00

Mathematics, 01.07.2019 16:00

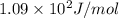
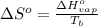
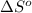 = change in entropy of vaporization = ?
= change in entropy of vaporization = ?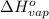 = change in enthalpy of vaporization = 40.7 kJ/mol
= change in enthalpy of vaporization = 40.7 kJ/mol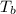 = boiling point temperature of water =
= boiling point temperature of water =