

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Two nitro no2 groups are chemically bonded to a patch of surface. they can't move to another location on the surface, but they can rotate (see sketch at right). it turns out that the amount of rotational kinetic energy each no2 group can have is required to be a multiple of ε, where =ε×1.010−24 j. in other words, each no2 group could have ε of rotational kinetic energy, or 2ε, or 3ε, and so forth — but it cannot have just any old amount of rotational kinetic energy. suppose the total rotational kinetic energy in this system is initially known to be 32ε. then, some heat is removed from the system, and the total rotational kinetic energy falls to 18ε. calculate the change in entropy. round your answer to 3 significant digits, and be sure it has the correct unit symbol.
Answers: 2

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 23.06.2019 01:00
The primary products of complete combustion of fossil fuels are a. carbon dioxide and water b. methane and water c. carbon monoxide and water d. carbon dioxide and carbon monoxide
Answers: 1
You know the right answer?
Given the standard enthalpy changes for the following two reactions:
(1) n2(g) + 2o2(g)2no2(...
(1) n2(g) + 2o2(g)2no2(...
Questions

Mathematics, 04.12.2020 01:00


Mathematics, 04.12.2020 01:00

History, 04.12.2020 01:00


Chemistry, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00

History, 04.12.2020 01:00

History, 04.12.2020 01:00




Mathematics, 04.12.2020 01:00

Mathematics, 04.12.2020 01:00

Biology, 04.12.2020 01:00





Social Studies, 04.12.2020 01:00

 ...... ΔH° = 66.4 kJ
...... ΔH° = 66.4 kJ
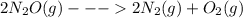 ......ΔH° = -164.2 kJ
......ΔH° = -164.2 kJ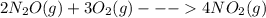 ......ΔH° = _________?
......ΔH° = _________?

