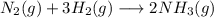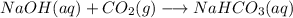Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3
You know the right answer?
Which of the following chemical reactions has positive entropy change?
a. n2(g) + 3 h2(g) \l...
a. n2(g) + 3 h2(g) \l...
Questions




Mathematics, 19.10.2019 09:20

English, 19.10.2019 09:20

Physics, 19.10.2019 09:20



Mathematics, 19.10.2019 09:20


Mathematics, 19.10.2019 09:20




Mathematics, 19.10.2019 09:20


Mathematics, 19.10.2019 09:20

Mathematics, 19.10.2019 09:20


Mathematics, 19.10.2019 09:20


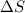 is positive when randomness increases and
is positive when randomness increases and