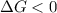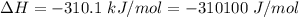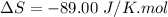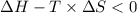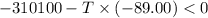Chemistry, 26.11.2019 03:31 Rileyb101207
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reaction in which δh = −310.1 kj/mol and δs = −89.00 j/k · mol, determine the temperature (in °c) below which the reaction is spontaneous.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 22.06.2019 18:40
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 06:30
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3
You know the right answer?
Areaction will be spontaneous only at low temperatures if both δh and δs are negative. for a reactio...
Questions

Mathematics, 21.05.2021 06:30



Mathematics, 21.05.2021 06:30


Mathematics, 21.05.2021 06:30




Mathematics, 21.05.2021 06:30








Mathematics, 21.05.2021 06:30

English, 21.05.2021 06:30

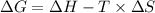
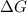 is the change in the Gibbs free energy.
is the change in the Gibbs free energy.
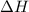 is the enthalpy change of the reaction.
is the enthalpy change of the reaction.
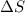 is the change in entropy.
is the change in entropy.