Chemistry, 26.11.2019 03:31 ashtonrieper1132
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 17. g of octane is mixed with 93.0 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 13:20
Determine which intermolecular forces are the dominant (strongest) forces for a pure sample of each of the following molecules by placing the molecules into the correct bins. drag the appropriate molecular formula to their respective bins.
Answers: 3

Chemistry, 22.06.2019 06:00
Compare and contrast physical changes with chemical changes.
Answers: 3


Chemistry, 22.06.2019 20:00
I’m an electrically neutral atomic any element, there are equal numbers of
Answers: 2
You know the right answer?
Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . s...
Questions


Mathematics, 12.12.2020 16:20





Mathematics, 12.12.2020 16:20



Mathematics, 12.12.2020 16:20

Mathematics, 12.12.2020 16:20

Physics, 12.12.2020 16:20


History, 12.12.2020 16:20

History, 12.12.2020 16:20

Biology, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20


Mathematics, 12.12.2020 16:20

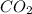
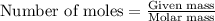
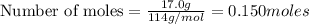
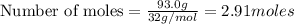
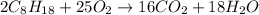
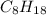 require 25 moles of
require 25 moles of 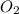
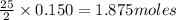 of
of 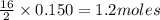 of
of