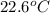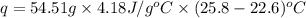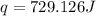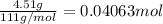Chemistry, 26.11.2019 03:31 juicecarton
When 4.51 g of cacl2 dissolved in 50.00 ml of water in a coffee cup calorimeter, the temperature of the solution rose from 22.6â°c to 25.8â°c.
specific heat of the solution is equal to the specific heat of water = 4.18 j/gâºc.
density of the solution is equal to the density of water = 1.00 g/ml.
what is qsolution?
what is qreaction ?
what is îhrxn in kj/mol of cacl2 ?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 14:30
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1
You know the right answer?
When 4.51 g of cacl2 dissolved in 50.00 ml of water in a coffee cup calorimeter, the temperature of...
Questions



Physics, 05.07.2019 08:00

Social Studies, 05.07.2019 08:00


English, 05.07.2019 08:00


Mathematics, 05.07.2019 08:00


Biology, 05.07.2019 08:00

Physics, 05.07.2019 08:00

Chemistry, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00

Mathematics, 05.07.2019 08:00




Biology, 05.07.2019 08:00


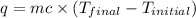
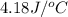
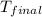 = final temperature =
= final temperature = 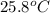
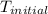 = initial temperature =
= initial temperature =