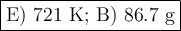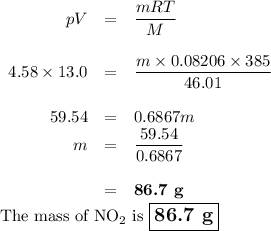So
25
7.) a syringe initially holds a sample of gas with a volume of 285 ml at 355 k and...

Chemistry, 26.11.2019 03:31 dpchill5232
So
25
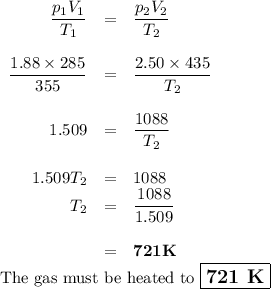
7.) a syringe initially holds a sample of gas with a volume of 285 ml at 355 k and 1.88 atm. to
what temperature must the gas in the syringe be heated/cooled in order to have a volume of 435
ml at 2.50 atm?
a) 139 k
b) 572 k
c) 175 k
d) 466 k
e) 721 k
8.) what mass of no2 is contained in a 13.0 l tank at 4.58 atm and 385 k?
a) 18.8 g
b) 86.7 g
c) 24.4 g
d) 53.1 g
e) 69.2 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 01:00
Na chemical reaction, activation energy increases the of the reactants. this outcome causes the particles to collide, which results in the of new products.
Answers: 2

You know the right answer?
Questions


Mathematics, 25.05.2021 04:40


Mathematics, 25.05.2021 04:40


Chemistry, 25.05.2021 04:40

Computers and Technology, 25.05.2021 04:40



Mathematics, 25.05.2021 04:40

Mathematics, 25.05.2021 04:40


Mathematics, 25.05.2021 04:40