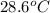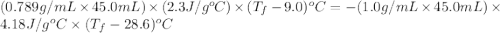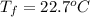Chemistry, 26.11.2019 02:31 kylemartinez13
If 45.0 ml of ethanol (density=0.789 g/ml) initially at 9.0 c is mixed with 45.0 ml of water (density=1.0 g/ml) initially at 28.6 c in an insulated beaker, and assuming that no heat is lost, what is the final temperature of the mixture?
i tried many times in trying to get the answer, but i keep getting it wrong. i appreciate who answers this writes it step by step. you.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
1. baking powder is a 1: 1 molar mixture of cream of tartar (khc4h4o6) and baking soda (nahco3). a recipe calls for two teaspoons (a total of 8.0 grams) of cream of tartar. how much baking soda must be added for both materials to react completely?
Answers: 2


Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 17:30
Air can be considered a mixture. which statement does not explain why?
Answers: 1
You know the right answer?
If 45.0 ml of ethanol (density=0.789 g/ml) initially at 9.0 c is mixed with 45.0 ml of water (densit...
Questions


History, 05.12.2020 04:00


History, 05.12.2020 04:00

Physics, 05.12.2020 04:00

English, 05.12.2020 04:00



English, 05.12.2020 04:10


Geography, 05.12.2020 04:10

Mathematics, 05.12.2020 04:10

Mathematics, 05.12.2020 04:10

Mathematics, 05.12.2020 04:10

Mathematics, 05.12.2020 04:10



English, 05.12.2020 04:10

Mathematics, 05.12.2020 04:10

Mathematics, 05.12.2020 04:10

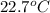
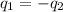
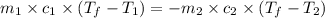
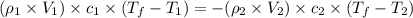
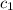 = specific heat of ethanol =
= specific heat of ethanol = 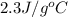
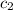 = specific heat of water =
= specific heat of water = 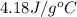
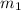 = mass of ethanol
= mass of ethanol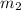 = mass of water
= mass of water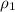 = density of ethanol = 0.789 g/mL
= density of ethanol = 0.789 g/mL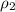 = density of water = 1.0 g/mL
= density of water = 1.0 g/mL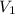 = volume of ethanol = 45.0 mL
= volume of ethanol = 45.0 mL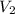 = volume of water = 45.0 mL
= volume of water = 45.0 mL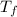 = final temperature of mixture = ?
= final temperature of mixture = ?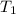 = initial temperature of ethanol =
= initial temperature of ethanol = 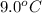
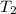 = initial temperature of water =
= initial temperature of water =