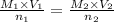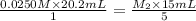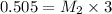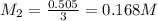Ferris & mona used the orp sensor to titrate a ferrous ammonium sulfate solution, (nh4)2fe(so4)2 with kmno4 titrant.
they titrated a 15.00 ml aliquot of the fe+2 solution with 0.0250 m mno4- solution and determined that the equivalence point was at 20.2 ml.
what is the molarity of the fe+2 solution? 5 fe+2(aq) + mno4-(aq) + 8 h+(aq) â 5 fe+3(aq) + mn+2(aq) + 4 h2oselect one: a. 0.168 mb. 0.0928 mc. 0.0337 md. 0.673 m

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 05:30
Compare and contrast physical changes with chemical changes.
Answers: 1

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
Ferris & mona used the orp sensor to titrate a ferrous ammonium sulfate solution, (nh4)2fe(so4)...
Questions

History, 15.01.2021 20:10

English, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10

Chemistry, 15.01.2021 20:10

History, 15.01.2021 20:10


English, 15.01.2021 20:10

Mathematics, 15.01.2021 20:10




Mathematics, 15.01.2021 20:10



History, 15.01.2021 20:10




English, 15.01.2021 20:10