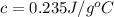Achemist carefully measures the amount of heat needed to raise the temperature of a 894.0 g sample of a pure substance from -5.8c to 17.5c. the experiment shows that 4.90kjs of heat are needed. what can the chemist report for the specific heat capacity of the substance? (round your answer to 3 significant digits.)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 18:30
How many moles of bromine are needed to produce 3.23 moles of potassium bromide
Answers: 1

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
Achemist carefully measures the amount of heat needed to raise the temperature of a 894.0 g sample o...
Questions



Chemistry, 24.04.2021 06:10

Mathematics, 24.04.2021 06:10

Business, 24.04.2021 06:10

Mathematics, 24.04.2021 06:10



Mathematics, 24.04.2021 06:10






Mathematics, 24.04.2021 06:10


Mathematics, 24.04.2021 06:10

Chemistry, 24.04.2021 06:10


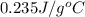
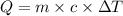
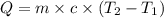
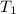 = initial temperature =
= initial temperature = 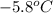
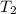 = final temperature =
= final temperature = 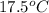
![4900J=894.0g\times c\times [17.5-(-5.8)]^oC](/tpl/images/0390/7580/b9d66.png)