Chemistry, 26.11.2019 01:31 billlyyyyyyyyyy
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is performing 855 j of work on the surroundings. 9.00 x 102 kj 64.1 kj -9.00 x 102 kj -64.1 kj -65.9 kj

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 23:00
What is the name of the enzyme that forms at the start of transcription?
Answers: 1

Chemistry, 23.06.2019 01:30
Ariver current has a velocity of 5km/h relative to the shore, and a boat moves in the same direction as the current at 5 km/h relative to the river. how can the velocity of the boat relative to the shore be calculated?
Answers: 1
You know the right answer?
Calculate the change internal energy (δe) for a system that is giving off 65.0 kj of heat and is per...
Questions





Physics, 24.09.2020 08:01

English, 24.09.2020 08:01


English, 24.09.2020 08:01



English, 24.09.2020 08:01

Social Studies, 24.09.2020 08:01







Mathematics, 24.09.2020 08:01

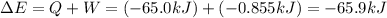
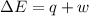
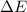 =Change in internal energy
=Change in internal energy
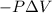 {Work is done by the system is negative as the final volume is greater than initial volume}
{Work is done by the system is negative as the final volume is greater than initial volume}